1.3.4: Other Units for Solution Concentrations
Learning Objectives
- Define the concentration units of mass percentage, volume percentage, mass-volume percentage, parts-per-million (ppm), and parts-per-billion (ppb)
- Perform computations relating a solution’s concentration and its components’ volumes and/or masses using these units
In the previous section, we introduced molarity, a very useful measurement unit for evaluating the concentration of solutions. However, molarity is only one measure of concentration. In this section, we will introduce some other units of concentration that are commonly used in various applications, either for convenience or by convention.
Mass Percentage
Earlier in this chapter, we introduced percent composition as a measure of the relative amount of a given element in a compound. Percentages are also commonly used to express the composition of mixtures, including solutions. The mass percentage of a solution component is defined as the ratio of the component’s mass to the solution’s mass, expressed as a percentage:
[latex]\text{mass percentage} = \dfrac{\text{mass of component}}{\text{mass of solution}} \times100\% \label{3.5.1}[/latex]
We are generally most interested in the mass percentages of solutes, but it is also possible to compute the mass percentage of solvent.
Mass percentage is also referred to by similar names such as percent mass, percent weight, weight/weight percent, and other variations on this theme. The most common symbol for mass percentage is simply the percent sign, %, although more detailed symbols are often used including %mass, %weight, and (w/w)%. Use of these more detailed symbols can prevent confusion of mass percentages with other types of percentages, such as volume percentages (to be discussed later in this section).
Mass percentages are popular concentration units for consumer products. The label of a typical liquid bleach bottle (Figure 1.3.4.1) cites the concentration of its active ingredient, sodium hypochlorite ([latex]\ce{NaOCl}[/latex]), as being 7.4%. A 100.0-g sample of bleach would therefore contain 7.4 g of [latex]\ce{NaOCl}[/latex].

Example 1.3.4.1: Calculation of Percent by Mass
A 5.0-g sample of spinal fluid contains 3.75 mg (0.00375 g) of glucose. What is the percent by mass of glucose in spinal fluid?
Solution
The spinal fluid sample contains roughly 4 mg of glucose in 5000 mg of fluid, so the mass fraction of glucose should be a bit less than one part in 1000, or about 0.1%. Substituting the given masses into the equation defining mass percentage yields:
[latex]\mathrm{\%\,glucose=\dfrac{3.75\;mg \;glucose \times \frac{1\;g}{1000\; mg}}{5.0\;g \;spinal\; fluid}=0.075\%} \nonumber[/latex]
The computed mass percentage agrees with our rough estimate (it’s a bit less than 0.1%).
Note that while any mass unit may be used to compute a mass percentage (mg, g, kg, oz, and so on), the same unit must be used for both the solute and the solution so that the mass units cancel, yielding a dimensionless ratio. In this case, we converted the units of solute in the numerator from mg to g to match the units in the denominator. We could just as easily have converted the denominator from g to mg instead. As long as identical mass units are used for both solute and solution, the computed mass percentage will be correct.
Exercise 1.3.4.1
A bottle of a tile cleanser contains 135 g of [latex]\ce{HCl}[/latex] and 775 g of water. What is the percent by mass of [latex]\ce{HCl}[/latex] in this cleanser?
- Answer
-
14.8%
Example 1.3.4.2: Calculations using Mass Percentage
“Concentrated” hydrochloric acid is an aqueous solution of 37.2% [latex]\ce{HCl}[/latex] that is commonly used as a laboratory reagent. The density of this solution is 1.19 g/mL. What mass of [latex]\ce{HCl}[/latex] is contained in 0.500 L of this solution?
Solution
The HCl concentration is near 40%, so a 100-g portion of this solution would contain about 40 g of HCl. Since the solution density isn’t greatly different from that of water (1 g/mL), a reasonable estimate of the HCl mass in 500 g (0.5 L) of the solution is about five times greater than that in a 100 g portion, or [latex]\mathrm{5 \times 40 = 200\: g}[/latex]. To derive the mass of solute in a solution from its mass percentage, we need to know the corresponding mass of the solution. Using the solution density given, we can convert the solution’s volume to mass, and then use the given mass percentage to calculate the solute mass. This mathematical approach is outlined in this flowchart:
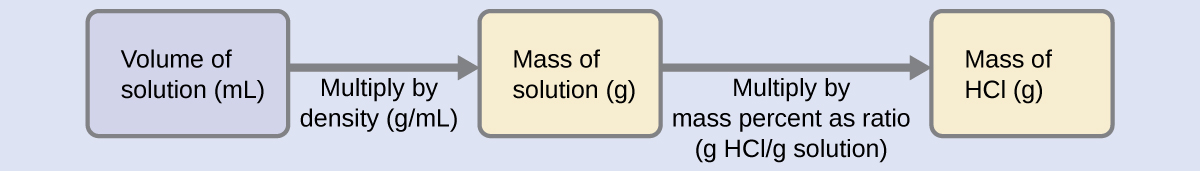
For proper unit cancellation, the 0.500-L volume is converted into 500 mL, and the mass percentage is expressed as a ratio, 37.2 g HCl/g solution:
[latex]\mathrm{500\; mL\; solution \left(\dfrac{1.19\;g \;solution}{mL \;solution}\right) \left(\dfrac{37.2\;g\; HCl}{100\;g \;solution}\right)=221\;g\; HCl} \nonumber[/latex]
This mass of HCl is consistent with our rough estimate of approximately 200 g.
Exercise 1.3.4.2
What volume of concentrated HCl solution contains 125 g of HCl?
- Answer
-
282 mL
Volume Percentage
Liquid volumes over a wide range of magnitudes are conveniently measured using common and relatively inexpensive laboratory equipment. The concentration of a solution formed by dissolving a liquid solute in a liquid solvent is therefore often expressed as a volume percentage, %vol or (v/v)%:
[latex]\text{volume percentage} = \dfrac{\text{volume solute}}{\text{volume solution}} \times100\% \label{3.5.2}[/latex]
Example 1.3.4.3: Calculations using Volume Percentage
Rubbing alcohol (isopropanol) is usually sold as a 70%vol aqueous solution. If the density of isopropyl alcohol is 0.785 g/mL, how many grams of isopropyl alcohol are present in a 355 mL bottle of rubbing alcohol?
Solution
Per the definition of volume percentage, the isopropanol volume is 70% of the total solution volume. Multiplying the isopropanol volume by its density yields the requested mass:
[latex]\text {355 mL solution}(\frac{\text{70 mL isopropyl alcohol}}{\text{100 mL solution}})(\frac{\text{0.785 g isopropyl alcohol}}{\text{1 mL isopropyl alcohol}})=\text{195 g isopropyl alcohol} \nonumber[/latex]
Exercise 1.3.4.3
Wine is approximately 12% ethanol ([latex]\ce{CH_3CH_2OH}[/latex]) by volume. Ethanol has a molar mass of 46.06 g/mol and a density 0.789 g/mL. How many moles of ethanol are present in a 750-mL bottle of wine?
- Answer
-
1.5 mol ethanol
Mass-Volume Percentage
“Mixed” percentage units, derived from the mass of solute and the volume of solution, are popular for certain biochemical and medical applications. A mass-volume percent is a ratio of a solute’s mass to the solution’s volume expressed as a percentage. The specific units used for solute mass and solution volume may vary, depending on the solution. For example, physiological saline solution, used to prepare intravenous fluids, has a concentration of 0.9% mass/volume (m/v), indicating that the composition is 0.9 g of solute per 100 mL of solution. The concentration of glucose in blood (commonly referred to as “blood sugar”) is also typically expressed in terms of a mass-volume ratio. Though not expressed explicitly as a percentage, its concentration is usually given in milligrams of glucose per deciliter (100 mL) of blood (Figure 1.3.4.2).

Parts per Million and Parts per Billion
Very low solute concentrations are often expressed using appropriately small units such as parts per million (ppm) or parts per billion (ppb). Like percentage (“part per hundred”) units, ppm and ppb may be defined in terms of masses, volumes, or mixed mass-volume units. There are also ppm and ppb units defined with respect to numbers of atoms and molecules.
The mass-based definitions of ppm and ppb are given here:
[latex]\text{ppm}=\dfrac{\text{mass solute}}{\text{mass solution}} \times 10^6\; \text{ppm} \label{3.5.3A}[/latex]
[latex]\text{ppb}=\dfrac{\text{mass solute}}{\text{mass solution}} \times 10^9\; \text{ppb} \label{3.5.3B}[/latex]
Both ppm and ppb are convenient units for reporting the concentrations of pollutants and other trace contaminants in water. Concentrations of these contaminants are typically very low in treated and natural waters, and their levels cannot exceed relatively low concentration thresholds without causing adverse effects on health and wildlife. For example, the EPA has identified the maximum safe level of fluoride ion in tap water to be 4 ppm. Inline water filters are designed to reduce the concentration of fluoride and several other trace-level contaminants in tap water (Figure 1.3.4.3).

Example 1.3.4.4: Parts per Million and Parts per Billion Concentrations
According to the EPA, when the concentration of lead in tap water reaches 15 ppb, certain remedial actions must be taken. What is this concentration in ppm? At this concentration, what mass of lead (μg) would be contained in a typical glass of water (300 mL)?
Solution
The definitions of the ppm and ppb units may be used to convert the given concentration from ppb to ppm. Comparing these two unit definitions shows that ppm is 1000 times greater than ppb (1 ppm = 103 ppb). Thus:
[latex]\mathrm{15\; \cancel{ppb} \times \dfrac{1\; ppm}{10^3\;\cancel{ppb}} =0.015\; ppm} \nonumber[/latex]
The definition of the ppb unit may be used to calculate the requested mass if the mass of the solution is provided. However, only the volume of solution (300 mL) is given, so we must use the density to derive the corresponding mass. We can assume the density of tap water to be roughly the same as that of pure water (~1.00 g/mL), since the concentrations of any dissolved substances should not be very large. Rearranging the equation defining the ppb unit and substituting the given quantities yields:
[latex]\text{ppb}=\dfrac{\text{mass solute}}{\text{mass solution}} ×10^9\; \text{ppb} \nonumber[/latex]
[latex]\text{mass solute} = \dfrac{\text{ppb} \times \text{mass solution}}{10^9\;\text{ppb}} \nonumber[/latex]
[latex]\text{mass solute}=\mathrm{\dfrac{15\:ppb×300\:mL×\dfrac{1.00\:g}{mL}}{10^9\:ppb}=4.5 \times 10^{-6}\;g} \nonumber[/latex]
Finally, convert this mass to the requested unit of micrograms:
[latex]\mathrm{4.5 \times 10^{−6}\;g \times \dfrac{1\; \mu g}{10^{−6}\;g} =4.5\; \mu g} \nonumber[/latex]
Exercise 1.3.4.4
A 50.0-g sample of industrial wastewater was determined to contain 0.48 mg of mercury. Express the mercury concentration of the wastewater in ppm and ppb units.
- Answer
-
9.6 ppm, 9600 ppb
Summary
In addition to molarity, a number of other solution concentration units are used in various applications. Percentage concentrations based on the solution components’ masses, volumes, or both are useful for expressing relatively high concentrations, whereas lower concentrations are conveniently expressed using ppm or ppb units. These units are popular in environmental, medical, and other fields where mole-based units such as molarity are not as commonly used.
Glossary
- mass percentage
- ratio of solute-to-solution mass expressed as a percentage
- mass-volume percent
- ratio of solute mass to solution volume, expressed as a percentage
- parts per billion (ppb)
- ratio of solute-to-solution mass multiplied by 109
- parts per million (ppm)
- ratio of solute-to-solution mass multiplied by 106
- volume percentage
- ratio of solute-to-solution volume expressed as a percentage
